SEEFFOR 4 (1): 3-12
DOI: http://dx.doi.org/10.15177/seefor.13-01
Original scientific paper
Lead Tolerance and Accumulation in White Poplar Cultivated In Vitro
Branislav Kovačević 1*, Dragana Miladinović 2, Saša Orlović 1, Marina Katanić 1, Marko Kebert 1,
Jovana Kovinčić 3
1 University of Novi Sad, Institute of Lowland Forestry and Environment, Novi Sad, Serbia
2 Institute of Field and Vegetable Crops, Novi Sad, Serbia
3 University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia
* Corresponding author: e-mail:
Citation:
KOVAČEVIĆ B, MILADINOVIĆ D, ORLOVIĆ S, KATANIĆ M, KEBERT M, KOVINČIĆ J 2013 Lead Tolerance and Accumulation in White Poplar Cultivated In Vitro. South-east Eur for 4 (1): 3-12. DOI: http://dx.doi.org/10.15177/seefor.13-01
Cited by: CrossRef Google Scholar
Abstract
Background and Purpose: This paper analyses the lead tolerance and accumulation in white poplar genotypes in vitro, in order to optimize genotype evaluation and other procedures in their implementation in phytoremediation projects and landscaping in areas endangered by lead accumulation.
Material and Methods: The lead tolerance and accumulation of five white poplar genotypes after 35 days in vitro cultivation on media supplemented with lead was examined. The following Pb(NO3)2 concentrations were used: 0, 10-6, 10-5, 10-4 and 10-3 M. Tolerance analysis (described by tolerance indices) was based on morphological parameters, biomass accumulation and the content of photosynthetic pigments, while lead accumulation was described by shoot lead accumulation and shoot lead content.
Results and Conclusions: The chosen lead concentrations appeared not to be lethal. Moreover, the obtained results showed that the tested lead concentrations had a positive effect on: number of formed roots, shoot moisture content and shoot height. The best differentiation among the examined genotypes was gained by the tolerance index based on the shoot height on 10-4 M Pb(NO3)2. The shoot lead accumulation and shoot lead content significantly increased on 10-4 and 10-3 M Pb(NO3)2 media. Thus, the concentration of 10-4 M Pb(NO3)2 is recommended for further research. Two examined genotypes of horticultural value (LCM and LBM) achieved a significantly higher lead shoot content compared to the wide spread genotype “Villafranca” (almost 200% and 125% higher, respectively).
Keywords: Populus alba, tissue culture, phytoextraction
Abbreviations
ACM - Aspen culture medium; BAP 6 - Benzylaminopurine; NAA α - Naphthalene acetic acid; Pb(NO3)2 - Lead nitrate; Chl - chlorophyll; AAS - Atomic Absorption Spectrophotometer
INTRODUCTION
In recent years, the ecosystem and human habitable zones have been significantly polluted by various heavy metals. Heavy metals are potentially harmful to human health, as well as to plants and other living beings in general [1].
The toxic effect of most heavy metals is caused by their bonding to SH protein groups leading to the inhibition of enzyme activity and compromising their structure. They also substitute essential elements in biomolecules causing their deficiency [2]. High concentrations of metal ions in the soil limit the assimilation of important micro- and macronutrients by plants [3, 4].
Lead is one of the most toxic heavy metals [5] and a major pollutant in both terrestrial and aquatic ecosystems [6]. It affects enzyme activity and inhibits electron transport during oxidative phosphorylation, stimulating the formation of free radicals and reactive oxygen species resulting in oxidative stress [7, 8]. Lead naturally occurs in the soil but its content may be greatly increased by human activities [7]. River sediments also receive significant anthropogenic loads of metals from both point and nonpoint sources [9].
The research on the influence of different plant species on the contaminated soils and underground water started in the early 1980s [10, 11, 12]. The technology of using plants to remove heavy metals from the substrate is known as phytoremediation. Trees were suggested as a low-cost, sustainable and ecologically sound solution for the remediation of heavy metal-contaminated land [13], especially by phytoextraction [14].
Poplars are often used in phytoremediation due to their fast growth, adaptability, a well-developed root system that reaches underground waters, and the ability to transpire considerable amounts of water [15]. Poplars are not able to match hiperaccumulators in the heavy metal accumulation, but their main advantage is in the large biomass production [13] and a relativly high quantity of extracted metal per plant [14]. White poplars (section Leuce Duby.) in particular, are interesting for their high tolerance to arid conditions and their implementation in horticulture and landscaping, especially the genotypes with a pyramidal tree shape [16, 17]. The in vitro culture of tree species offers a rapid instrument to produce the clonal planting stock, but it may also facilitate studies on the effects of elevated levels of heavy metals on plant performance and the selection of metal-tolerant genotypes. Developmental and molecular data obtained by Castiglione et al. [18] in the research of white poplars suggested that the in vitro model was a sensitive and reliable system to study heavy metal stress responses. This is an important fact considering the difficulties in experimenting on large, long-lived organisms. The tolerance of white poplars to heavy metals, including lead, was tested in controlled conditions, and the differences among the genotypes were found [14], [19, 20, 21, 22, 23].
In this research, we studied different white poplar (Populus alba L.) genotypes in vitro for their lead tolerance based on morphological parameters, biomass accumulation and photosynthetic pigments content, as well as the lead accumulation in the above-ground plant parts.
The aim was to evaluate and select lead tolerant and accumulating genotypes, potentially interesting for lead phytoextraction on lead contaminated soils.
MATERIAL AND METHODS
Plant Material and Shoot Multiplication
Five white poplar genotypes, considered to be interesting for the biomass production, landscaping and horticulture were used in the research (Table 1).
TABLE 1. Examined white poplar genotypes
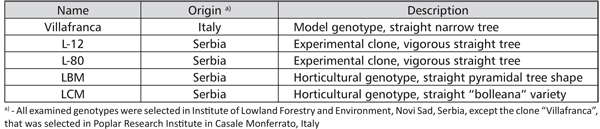
The genotype “Villafranca” was used as a standard, regarding the fact that it is widely used in biotechnology and in vitro studies of the heavy metal effect on white poplars [14, 15, 16, 17, 18]. ACM (Aspen Culture Medium), described by Ahuja [24], supplemented with 20 mg/l adenine-sulphate, 100 mg/l myo-inositole, 0.5 mg/l benzylaminopurine (BAP), 0.02 mg/l a-naphthaleneacetic acid (NAA), 9 g/l of agar and 20 g/l of sucrose, pH 5.5, was used for the shoot multiplication. Shoots of all five tested genotypes were multiplied through the shoot tip culture. The cultures were kept at 26±2oC in the white fluorescent light (3500 lux) with a 16 hour photoperiod and sub-cultured at 4-week intervals.
Lead Treatments
For the experiment, 1.5-2.0 cm long shoot tips of the previously multiplied shoots were placed on the rooting medium based on ACM with added 9 g/l of agar and 20 g/l of sucrose, pH 5.5, and supplemented with the following concentrations of lead in the form of Pb(NO3)2: 0 (as a Control), 10-6, 10-5, 10-4 and 10-3 M.
The cultures were kept in the same conditions as previously described. Three jars with five plants per jar were set for each combination of the genotype x lead concentration in three repetitions.
For pigment content determination, additional three jars with five shoots per jar were established per each combination of the genotype x lead concentration.
Lead Tolerance Assessment
After 35 days of cultivation, the following morphological traits were determined: the number of roots per shoot and the height of the shoot. The data for the number of roots was transformed by square transformation (![]() ) in order to meet the normal distribution of frequencies.
) in order to meet the normal distribution of frequencies.
The following traits describing biomass were determined: fresh shoot mass per plant, dry shoot mass per plant and the shoot water content. For the dry biomass calculation fifteen rootless shoots tips were dried at 100°C for 24 h and weighted. The fresh and dry biomass production was calculated as a difference between the fresh and dry biomass at the beginning and at the end of the experiment.
The concentration of chloroplast pigments: chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoids, was determined spectrophotometrically [25]. The Chl a+b and chlorophyll a/b ratios were calculated.
The toxicity of the applied lead concentration and differences in lead tolerance among the examined genotypes were evaluated by tolerance indices. The tolerance index (TI) was calculated according to [26], as a ratio between the value of a parameter on the medium with a particular lead concentration (Xc(Pb)) and the value obtained on the Control (XControl):
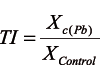
Tolerance indices were calculated for morphological traits, dry shoot biomass production traits and photosynthetic pigments content parameters.
Lead Accumulation Assessment
In order to determine lead accumulation i.e. the lead concentration in the dry biomass (mg/kg), samples were mineralized by wet ashing in the microwave digester and 65% HNO3 and 30% H2O2 (5:1 v/v). The lead content in a sample was detemined by the atomic absorption sectrometry (AA 240FS Fast Sequental Atomic Absorption Spectrometer, Varian, Australia).For the evaluation of the lead shoot accumulation of examined genotypes, shoot lead accumulation (Pb2+ content per shoot dry mass) and shoot lead content (calculated as the product of mean dry biomass of shoot per plantlet and mean Pb2+ content per shoot dry mass) were determined.
Statistical Analysis
The whole experiment was designed as completely randomized. The obtained data was analyzed by ANOVA and ANCOVA, as well as the LSD test with STATISTICA 10 statistical program [27].
RESULTS
Morphological Characters
No considerable chlorosis, necrosis or decay of shoot tissue was observed, and the rooting was nearly 100% on all examined media. Only on the medium with 10-3M c(Pb(NO3)2) a partial darkening of roots was noticed. According to the results of ANOVA, differences among the genotypes had significant effect on the variation of the examined morphological traits (Table 2). The effect of lead concentration was significant for a number of roots and shoot height, while interaction genotype × lead concentration had no significant influence on any of the examined morphological traits. The controlled sources of variation had the lowest influence on the variation of the length of the longest root.
TABLE 2. Results of F-test for examined characters in white poplar genotypes on examined media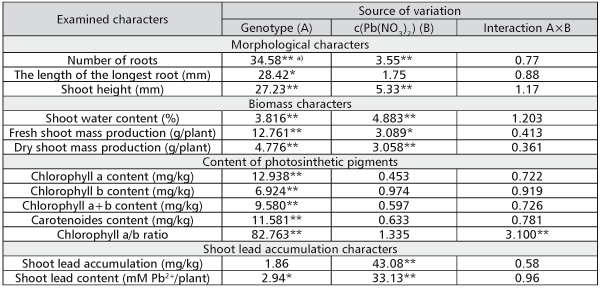
All examined lead concentrations significantly stimulated root formation. The most stimulative effect on the number of roots was observed in L-12 in the medium with 10-6 M c(Pb(NO3)2) and in L-80 in media with 10-4 M and 10-6 M c(Pb(NO3)2) (Table 3).
TABLE 3. Number of roots in white poplar on examined lead concentration in medium (LSD test)
The differences in tolerance indices for this trait and for the length of the longest root were mostly not significant, therefore indices based on these two traits were excluded from further tolerance evaluation (data not shown).
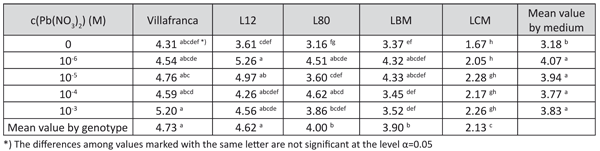
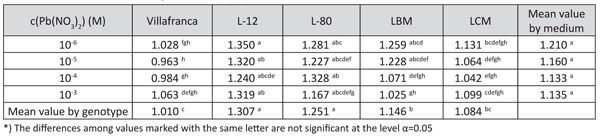
Shoot height showed a similar positive reaction to lead treatment as the number of roots (data not shown). The tolerance indices for this trait were mostly higher than 1, indicating a high tolerance of the examined clones to the presence of lead (Table 4). Tolerance indices for this trait differed significantly among the examined genotypes in all tested lead concentrations, with best results for genotypes L-12 and L-80.
TABLE 4. Tolerance index for shoot height of white poplar on examined lead concentration in medium (LSD test)
Biomass Characters
All biomass traits were significantly affected by both genotype and Pb concentration in the nutrient medium (Table 2).
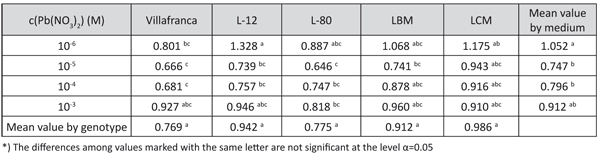
Generally, tolerance indices revealed the highest dry shoot mass production was achieved on the medium with 10-6 M Pb(NO3)2, followed by decrease on media with 10-5 M Pb(NO3)2 and 10-4 M Pb(NO3)2, and not significant increase in medium with 10-3 M Pb(NO3)2 (Table 5). Similar relations among treatments were found for fresh shoot mass production, but plants on all media supplemented with lead had a significantly higher shoot water content than in the Control (data not shown).
According to the tolerance index based on dry shoot mass production, the differences among the genotypes were only significant on the medium with 10-6 M Pb(NO3)2. The differences in tolerance index were rather low among the examined genotypes. The highest tolerance index for dry shoot mass production was observed in genotype L-12 (Table 5). The data for fresh shoot mass revealed similar relations among the genotypes (data not shown).
TABLE 5. Tolerance index for dry shoot mass production of white poplar on media with examined lead concentrations (LSD – test)
Influence of Rooting and Shoot Height on Biomass Traits
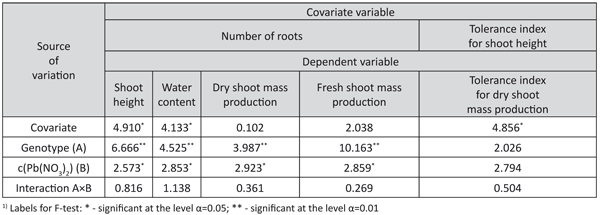
The analysis of the covariance showed that the number of roots had a significant influence on the shoots’ height and water content (Table 6). The influence of the number of roots on dry shoot mass production was not significant, but a significant influence of the tolerance index for shoot height on the variation of the tolerance indices of dry shoot mass production was observed.
The effect of the differences among the genotypes on this trait was not significant.
TABLE 6. F-test from the analysis of covariance for some characters of interest for lead tolerance in white poplar genotypes
Photosynthetic Pigments Content
According to the ANOVA results, genotype had a significant effect on the variation of the content of the examined photosynthetic pigments, as well as Chl a/b ratio, while the interaction genotype × lead concentration had a significant effect only on Chl a/b ratio (Table 2).
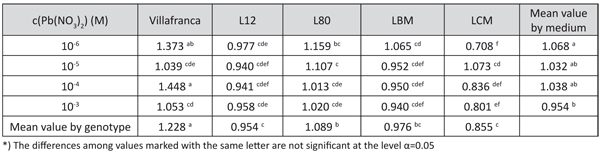
The content of all of the studied pigments, the base tolerance index, was not significantly affected by the controlled sources of variation. However, the tolerance index based on Chl a/b was also under a significant influence of the genotype and genotype × lead concentration (data not shown). The highest tolerance indices were observed in “Villafranca” and the lowest in LCM and L-12. The tolerance index gradually decreased with the increase of lead concentration, suggesting the increase of the chlorophyll b share (Table 7).
TABLE 7. The effect of lead concentration in medium on the tolerance index for Chl a/b ratio in white poplar genotypes (LSD – test)
Shoot Lead Accumulation and Content
In contrast to other traits, the variation of shoot lead content per plant and variation of lead content in dry shoot mass (shoot lead accumulation) were influenced principally by the lead concentration in the media. ANOVA showed that influence to be highly significant (Table 2).
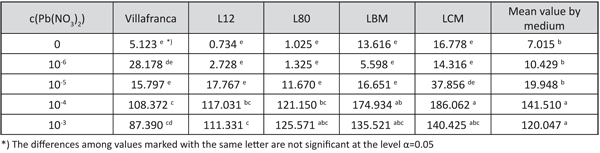
The shoot lead accumulation on the media with 10-4 and 10-3 M Pb(NO3)2 was significantly higher than on the media with other examined lead concentrations. The best differentiation in shoot lead accumulation among the examined genotypes was achieved with the concentration of 10-4 M Pb(NO3)2. The highest shoot lead accumulation on that medium was observed in genotype LCM, and the lowest in genotype “Villafranca” (Table 8).
TABLE 8. The lead content in dry shoot biomass (mg/kg) (shoot lead accumulation) in white poplar rooted shoots grown on examined growing media (LSD – test)
The shoot lead content per plant data was associated with the results of shoot lead accumulation. A considerable increase in shoot lead content was observed on the media with 10-4 and 10-3 M Pb(NO3)2. The shoot lead content in “Villafranca” plants on 10-4 M Pb(NO3)2was more than 2.5 times lower than in LBM and LCM on the same medium (Figure 1).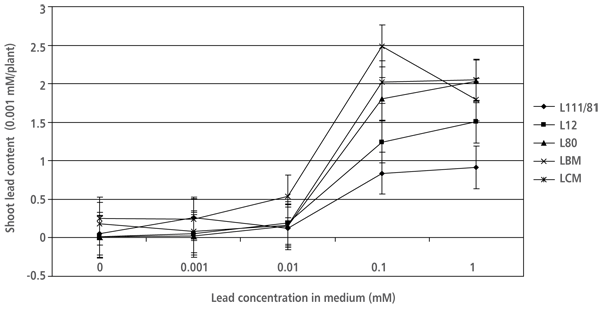
FIGURE 1. Shoot lead content (µM Pb2+/plant) in white poplar plantlets grown on examined growing media (SE derived from the results of ANOVA is marked)
DISCUSSION
The success of phytoextraction is mainly related to the ability of plants to tolerate the presence of a pollutant in the substrate and to accumulate it in their above-ground parts [14], [28]. Thus, high lead tolerance and accumulation were principal criteria in the evaluation of the genotypes in our research. Also, beside the selection of genotypes, the concentration of lead in the medium was evaluated in order to select the media that achieved the best differentiation among the genotypes, in order to determine the appropriate lead concentration for further in vitro tests.
Considering the tolerance of the examined genotypes, it may be said that there was no necrosis or decay of shoots on any media with lead, which indicates no significant lead pollution in the examined concentrations. This is consistent with the results obtained by Katanić et al. [21], who found no difference in leaf colour of white poplar shoots on the multiplication media with 0, 10-5 and 10-4 Pb·EDTA. However, in our experiment, root necrosis was present on the medium with 10-3 M Pb(NO3)2. This is in accordance with the results of Di Lonardo et al. [14], who found roots of white poplar genotypes in vitro to be more sensitive to heavy metals than shoots. The absence of a significant decrease of the content of photosynthetic pigments also confirmed that the examined lead concentrations had not severe inhibitory effects on the plants’ vitality.
The tolerance indices based on morphological, biomass and photosynthetic pigment content traits also suggested a high tolerance of the examined genotypes on the applied lead concentrations.
Lead treatment had a stimulative effect on the number of roots. According to Seregin and Ivanov [7], low lead concentrations promoted root growth. However, on relatively high concentrations of 2.0 mM Pb(NO3)2, Bojarczuk [20] found an inhibitory effect of on shoot and root development on the calluses of hybrid aspen on the regeneration medium. However, there were no significant differences among the genotypes in the tolerance indices based on the number of roots, as well as the length of the longest root (data not shown).
The tolerance index for shoot height (mostly above 1), indicated the absence of inhibition, if not a stimulative effect of the examined lead concentrations on shoot growth, especially in the medium with the 10-6 M Pb(NO3)2. The strong influence of differences among the genotypes recommeded this index to be used in tolerance evaluation tests in the future. According to the analysis of the covariance, shoot height was significantly influenced by the number of roots that also significantly influenced the shoot water content. The number of roots had no significant effect on dry shoot mass production, while the tolerance index based on shoot height significantly influenced the tolerance index of dry shoot mass production. These results suggested that the tolerance index based on shoot height is a good indicator of the lead tolerance of the tested genotypes. The positive effect of the implementation of this index would also be the option to establish more efficient and larger lead tolerance tests for white poplar genotypes in vitro. As the tolerance index for shoot height provided the best differentiation among the genotypes on the media with 10-4 M Pb(NO3)2, this lead concentration may be recommended in further testing.
The decrease in dry shoot mass production was observed on lead concentrations higher than 10-6 M Pb(NO3)2. Kališova-Šprirochova et al. [19] reported a stimulative effect of 10-4 M Pb2+ on the total plant biomass accumulation in aspen rooted shoots in the liquid medium in vitro. However, Katanić et al. [21] found inhibitory effects on the fresh biomass of white poplar shoot tips grown on the multiplication medium supplemented with 10-4 M PbEDTA. The differentiation among the genotypes in the tolerance index based on dry shoot biomass production was low. Significant differences were recorded only on 10-6 M Pb(NO3)2 where the genotype L-12 had a significantly higher index than in the standard genotype “Villafranca”.
There was a low effect of lead concentration on the variation of photosynthetic pigment traits, except for the Chl a/b ratio, where the influence of the interaction genotype × medium on its variation was significant. Ewais [29] found a statistically significant decrease of the content of photosynthetic pigments in leaves of three weed species grown in pots with 200 mg/kg (0.6 mM/kg) of Pb acetate. Also, Zengin and Munzuroglu [8] found a significant decrease on the chlorophyll content in leaves (about 10%) together with the increase of several oxidative stress indicators in the lead-treated seedlings of common bean. However, Sarvari et al. [30] found a mild increase of the chlorophyll content in hydroponically grown white poplars in the nutrient solution with 10-5 M Pb(NO3)2 and 10-4 M Pb(NO3)2, while 10-4 M Pb(NO3)2 had aninhibitory effect on the chlorophyll content in cucumber plants. Kalsila et al. [31] also found a slightly stimulative effect of lead acetate added to the substrate in the concentration 200 mg/kg of lead on the chlorophyll content in oat and barley leaves. In general, the tolerance index for Chl a/b ratio was higher than 1 at the low lead concentration (10-6 M Pb(NO3)2), but decreased at higher concentrations. According to Sarvari et al. [30] the increase of Chl a/b ratio in poplar leaves in hydroponics with 10 μM Pb2+ may be explained by the increase in the amount of core complexes. However, at a higher lead concentration (100 μM), the lowering of the Chl a/b ratio occured due to a stronger decrease in the amount of PSI and the relative stability of PSII. Kamel [32] found a higher Chl a/b ratio on 4.8 mM Pb2+ and lower in the 48 mM Pb2+ solution compared to the control in Vicia faba hydroponics after 96 hours of growth. The best differentiation among the examined genotypes, according to the tolerance index based on the Chl a/b ratio, was observed on media with 10-4 and10-3 M Pb(NO3)2. Regarding the results of Sarvari et al. [30], high values for “Villafranca” indicated a lower disturbance in chlorophyll synthesis and thus a higher lead tolerance by this parameter. However, the changes in tolerance indices based on the Chl a or Chl b content in shoot fresh mass may not be detected (data not shown), which additionally supported the conclusion of low differences among the examined genotypes in their lead tolerance.
The highest shoot lead accumulation and shoot lead content of the examined white poplar genotypes appeared on the media with 10-4 and 10-3 M Pb(NO3)2. The best differentiation among the genotypes was achieved on the 10-4 M Pb(NO3)2 medium. This is consistent with Sakan et al. [9] who found that shoot lead accumulation in the leaves of white poplar plants was not significant in hydroponics with 10-5 M Pb(NO3)2, but it was in hydroponics with 10-4 M Pb(NO3)2. These results suggested that the medium with 10-4 M Pb(NO3)2may be recommended to be applied in further tests on shoot lead accumulation in white poplar tissue cultures.
The widely-used genotype “Villafranca” was used as the standard [14], [18] in evaluation of the genotype tolerance to different lead concentrations, as well as the shoot lead accumulation. Lead tolerance was satisfying in the examined genotypes but the differences among them were rather low, in general. The mostly used tolerance indices in lead tolerance studies are based on the length of the longest root [33] or dry mass production [14], but in our research they gave a poor differentiation among the genotypes. The best differentiation was obtained by the tolerance index based on shoot height. According to this parameter, the most tolerant were genotypes L-80 and L-12.
On the other hand, lead accumulation in the above-ground parts appeared to be highly influenced by the differences among the genotypes, emphasizing the importance of shoot lead accumulation in the evaluation of the genotypes for the lead phytoextraction potential. The highest shoot lead accumulation on the medium with 10-4 M Pb(NO3)2 was achieved by the genotype LCM (cca. 70% higher compared to “Villafranca”).
The relation among the genotypes was similar for shoot lead content, but the difference between LCM and “Villafranca” was observed to be even higher (almost 200%). Beside LCM, similar results were observed also in the genotype LBM. Thus, as the tolerance of genotypes LCM and LBM was at the same level as most of the other genotypes and they achieved a high shoot lead accumulation, they may be recommended for further examination in lead phytoextraction plantations.
Considering the observed differences among the examined genotypes regarding lead tolerance and accumulation, in vitro tests may be utilized for selecting a group of candidate genotypes for lead phytoextraction projects. Watson et al. [34] and Pulford et al. [35] demonstrated in Salix sp. that the results obtained in hydroponics and in field are comparable. The general opinion is that the differences in the bioavailability of contaminants, the processes of pollutant uptake and metabolite distribution are likely to be substantial in tissue culture and field conditions. Thus, based on the results from tissue cultures, the response of plants to environmental contaminants may be predicted with a cost reduction of the subsequent conventional whole plant experiments [36, 37]. However, for the final evaluation of a particular genotype, further research should be done considering the lower availability of lead in soil, the higher juvenility of the in vitro material and the complexity of interactions between plants and their habitat.
CONCLUSIONS
According to the presented results, the following conclusions were drawn for the examined group of white poplar genotypes:
The tested lead concentrations had a positive effect on a number of formed roots, and by this trait on further on shoot moisture content and shoot height.
The tolerance index based on shoot height (significantly related to the tolerance index based on dry shoot mass production) and lead shoot content on a medium with 10-4 M Pb(NO3)2 were proposed to be used in further lead tolerance and accumulation research and testing.
Two examined genotypes of horticultural value (LCM and LBM) achieved a significantly higher lead shoot content compared to the wide-spread genotype “Villafranca” (almost 200% and 125% higher, respectively).
Acknowledgements
This paper was realized as a part of the project “Studying Climate Change and its Influence on the Environment: Impacts, Adaptation and Mitigation” (43007), financed by the Ministry of Education and Science of the Republic of Serbia within the framework of the integrated and interdisciplinary research for the period between 2011-2014.
REFERENCES
- ARORA M, KIRAN B, RANI S, RANI A, KAUR B, MITTAL N 2008 Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem 111 (4): 811–815. DOI: http://dx.doi.org/10.1016/j.foodchem.2008.04.049
- VAN ASSCHE F, CLIJSTERS H 1990 Effects of metals on enzyme activity in plants. Plant Cell Environ 13 (3): 195-206. DOI: http://dx.doi.org/10.1111/j.1365-3040.1990.tb01304.x
- BURZYŃSKI M, BUCZEK J 1998 Uptake and assimilation of ammonium ions by cucumber seedlings from solutions with different pH and addition of heavy metals. Acta Soc Bot Pol 76 (2): 197-200. DOI: http://dx.doi.org/10.5586/asbp.1998.023
- OLEKSYN J, KAROLEWSKI P, GIERTYCH MJ, WERNER A, TJOELKER MG, REICH PB 1996 Altered root growth and plant chemistry of Pinus sylvestris seedlings subjected to aluminium in nutrient solution. Trees 10 (3): 135-144
- ZHANG Y 2003 100 Years of Pb deposition and transport in soils in Champaign, Illinois, U.S.A. Water Air Soil Pollut 146 (1-4): 197–210. DOI: http://dx.doi.org/10.1023/A:1023957226204
- SHARMA P, DUBEY RS 2005 Lead toxicity in plants. Braz J Plant Physiol 17 (1): 35-52. DOI: http://dx.doi.org/10.1590/S1677-04202005000100004
- SEREGIN IV, IVANOV VB 2001 Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physl+ 48 (4): 523-544. DOI: http://dx.doi.org/10.1023/A:1016719901147
- ZENGIN FK, MUNZUROGLU O 2005 Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Bot 47/2: 157–164
- SAKAN SM, ĐOREVIĆ DS, MANOJLOVIĆ DD, POLIĆ PS 2009 Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manage 90 (11): 3382–3390. DOI: http://dx.doi.org/10.1016/j.jenvman.2009.05.013
- PIVETZ BE 2001 Ground water issue: Phytoremediation of contaminated soil and ground water at hazardous waste sites. U.S. Environmental Protection Agency, Technology Innovation Office, Office of Solid Waste and Emergency Response, Washington, DC, p 36
- BARCELOJ, POSCHENRIEDER C 2003 Phytoremediation: principles and perspectives. Contrib Sci 2 (3): 333-344
- GHOSH M, SINGH SP 2005 A review on phytoremediation of heavy metals and utilization of its byproducts. App Ecol Env Res 3 (1): 1-18
- PULFORD ID, WATSON C 2003 Phytoremediation of heavy metal-contaminated land by trees - a review. Environ Int 29 (4): 529-540. DOI: http://dx.doi.org/10.1016/S0160-4120(02)00152-6
- DI LONARDO S, CAPUANA M, ARNETOLI M, GABBRIELLI R, GONNELLI C 2011 Exploring the metal phytoremediation potential of three Populus alba L. clones using an in vitro screening. Environ Sci Pollut R 18 (1): 82-90. DOI: http://dx.doi.org/10.1007/s11356-010-0354-7
- AITCHISON EW, KELLEY SL, ALVAREZ PJJ, SCHOOR JL 2000 Phytoremediation of 1,4-dioxane by hybrid poplar trees. Water Environ Res 72 (3): 313-321. DOI: http://dx.doi.org/10.2175/106143000X137536
- EGGENS CF, LOUGHEED EC, HILTON RJ 1972 Rooting of hardwood cuttings of boleana poplar. Can J Plant Sci 52: 599-604. DOI: http://dx.doi.org/10.4141/cjps72-092
- KOVACEVIC B, ORLOVIC S, RONCEVIC S, MILADINOVIC D 2010 The effect of silver ion, 1-napthalene acetic acid and 6-benzylaminopurine on micropropagation of „Fastgiate“ tree shape variety Populus alba cl. LBM. Acta Hort 885: 197-202
- CASTIGLIONE S, FRANCHIN C, FOSSATI T, LINGUA G, TORRIGIANI P, BIONDI S 2007 High zinc concentrations reduce rooting capacity and alter metallothionein gene expression in white poplar (Populus alba L. cv. Villafranca). Chemosphere 67 (6): 1117-1126. DOI: http://dx.doi.org/10.1016/j.chemosphere.2006.11.039
- KALIŠOVA-ŠPIROCHOVA I, PUNČOCHAROVA J, KAFKA Z, KUBAL M, SOUDEK P, VANEK T 2003 Accumulation of heavy metals by in vitro cultures of plants. Water Air Soil Pollut 3 (3): 269-276. DOI: http://dx.doi.org/10.1023/A:1023933902452
- BOJARCZUK K 2004 Effect of toxic metals on the development of poplar (Populus tremula L. x P. alba L.) cultured in vitro. Pol J Environ Stud 13 (2): 115-120
- KATANIC M, PILIPOVIC A, ORLOVIC S, KRSTIC B 2007 The influence of lead on the shoot growth and concentration of photosynthetic pigments in leaves of the white poplar Populus alba clones in vitro. Topola (Poplar) 179-180: 15-24 (in Serbian with English summary)
- KATANIC M, PILIPOVIC A, ORLOVIC S, KRSTIC B, KOVACEVIC B, PEKEC S 2008 The influence of nickel, cadmium and lead on the growth of the white poplar clones’ shoots in vitro. In: Orlović S (ed) Proceedings of International scientific conference “Forestry in achieving millennium goals”, 13-15 November 2008, Novi Sad, Serbia, pp 443-446
- BITTSANSZKY A, KOMIVES T, GULLNER G, GYULAI G, KISS J, HESZKY L, RADIMSZKY L, RENNENBERG H 2005 Ability of transgenic poplars with elevated glutathione content to tolerate zinc2+ stress. Environment International 31 (2): 251-254. DOI: http://dx.doi.org/10.1016/j.envint.2004.10.001
- AHUJA MR 1984 A commercially feasible micropropagation method for aspen. Silvae Genet 32 (4-5): 174-176
- WETTSTEIN D 1957 Chlorophyll-letale und der submikroskopische formwechsel der plastiden. Exp Cell Res 12 (3): 427-506. DOI: http://dx.doi.org/10.1016/0014-4827(57)90165-9
- TURNER RC, MARSHAL C 1972 The accumulation of zinc by subcellular fractions of roots of Agrostis tenuis Sibth. in relation to zinc tolerance. New Phytol 71 (4): 671-676. DOI: http://dx.doi.org/10.1111/j.1469-8137.1972.tb01277.x
- STATSOFT INC. 2011 STATISTICA (data analysis software system), version 10
- HUANG JW, CHEN J, BERTI WR, CUNNINGHAM SD 1997 Phytoremediation of lead-contaminated soils: Role of synthetic chelates in lead phytoextraction. Environ Sci Technol 31 (3): 800-805. DOI: http://dx.doi.org/10.1021/es9604828
- EWAIS EA 1997 Effects of cadmium, nickel and lead on growth, chlorophyll content and proteins of weed. Biol plantarum 39 (3): 403-410. DOI: http://dx.doi.org/10.1023/A:1001084327343
- SARVARI E, GASPAR L, FODOR F, CSEH E, KROEPFL K, VARGA A, BARON M 2002 Comparison of the effect of Pb treatment on thylacoid development in poplar and cucumber plant. Acta Biol Szeged 46 (3-4): 163-165
- KAZNINA NM, LAIDINEN GF, TITOV AF, TALANOV AV 2005 Effect of lead on the photosynthetic apparatus of annual grasses. Biol Bull 32 (2): 147-150. DOI: http://dx.doi.org/10.1007/s10525-005-0022-5
- KAMEL HA 2008 Lead accumulation and its effect on photosynthesis and free amino acids in Vicia faba grown hydroponically. Aust J Basic Appl Sci 2 (3): 438-446
- MICHALAK E, WIERZBICKA M 1998 Differences in lead tolerance between Allium cepa plants developing from seeds and bulbs. Plant Soil 199 (2): 251-260. DOI: http://dx.doi.org/10.1023/A:1004321331708
- WATSON C, PULFORD ID, RIDDELL-BLACK D 2003 Screening of willow species for resistance to heavy metals: Comparison of performance in a hydroponics system and field trials. Int J Phytoremediat 5 (4): 351-365. DOI: http://dx.doi.org/10.1080/15226510309359042
- PULFORD ID, RIDDELL-BLACK D, STEWART C 2002 Heavy metal uptake by willow clones from sewage sludge-treated soil: the potential for phytoremediation. Int J Phytoremediat 4 (1): 59-72. DOI: http://dx.doi.org/10.1080/15226510208500073
- DORAN PM 2009 Application of plant tissue cultures in phytoremediation research: Incentives and limitations. Biotechnol Bioeng 103 (1): 60-76. DOI: http://dx.doi.org/10.1002/bit.22280
- CAPUANA M 2011 Heavy metals and woody plants - biotechnologies for phytoremediation. iForest 4: 7-15. DOI: http://dx.doi.org/10.3832/ifor0555-004
© 2015 by the Croatian Forest Research Institute. This is an Open Access paper distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0).


